B-Cell Acute Lymphoblastic Leukemia (B-ALL): A Detailed Guide
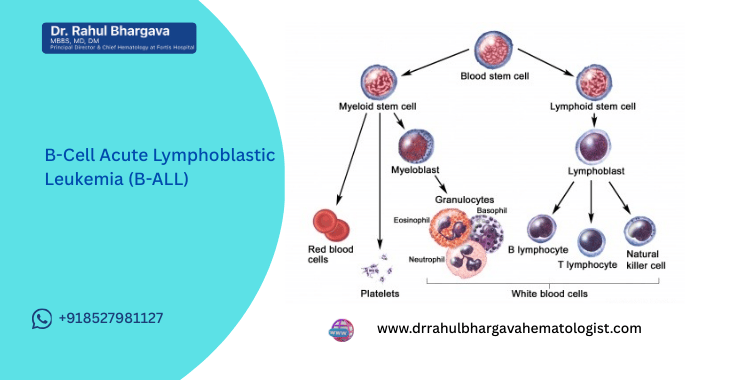
Table of Contents
B-cell Acute Lymphoblastic Leukemia (B-ALL) is a rapidly growing cancer of the blood and bone marrow. It affects the early (immature) forms of B-lymphocytes—cells responsible for fighting infections. Although fast-growing, it is one of the most treatable leukemias today due to advanced chemotherapy, immunotherapy, targeted drugs, and bone marrow transplantation.
As one of India’s most respected hematologists and bone marrow transplant specialists, Dr. Rahul Bhargava provides this comprehensive, medically accurate guide to help patients understand the disease, its treatment, and the latest advancements.
What Is B-Cell Acute Lymphoblastic Leukemia (B-ALL)?
B-ALL is a type of acute leukemia that arises from lymphoid stem cells in the bone marrow.
In B-ALL:
- Immature B-cells (lymphoblasts) multiply uncontrollably
- They crowd out normal blood-forming cells
- They can spill into the bloodstream
- They may spread to lymph nodes, liver, spleen, and the central nervous system
This leads to low blood counts (anemia, low platelets, low immunity) and a range of symptoms.
Causes & Risk Factors
The exact cause of B-ALL is usually unknown. However, research shows links with:
Genetic Factors
- Mutations in lymphoid stem cells
- Chromosome abnormalities (e.g., BCR-ABL1)
- Inherited syndromes (Down syndrome, Fanconi anemia)
Environmental Factors
- Previous exposure to high radiation
- Exposure to certain chemicals (rare and poorly proven)
Medical History
- Previous chemotherapy or radiation for another cancer
Immune System Conditions
- Rare immune deficiency syndromes
Most cases, however, occur without any clear trigger.
Symptoms of B-ALL
Symptoms usually appear suddenly, often over days to weeks.
Blood-related Symptoms
- Fatigue, weakness
- Pale skin (anemia)
- Shortness of breath
- Frequent infections
- Fever without clear cause
Bleeding Symptoms
- Easy bruising
- Bleeding gums
- Frequent nosebleeds
- Red skin spots (petechiae)
Bone Marrow Symptoms
- Bone pain (especially legs, hips)
- Joint pain
- Back pain
Other Symptoms
- Enlarged lymph nodes
- Liver or spleen enlargement
- Loss of appetite
- Weight loss
- Night sweats
- Headache or neurological changes (if CNS involved)
When Should You Consider Evaluation for B-ALL?
Early diagnosis dramatically improves survival.
You should consult a hematologist if any of these warning signs appear:
1. Persistent Fever for More Than 2 Weeks
Especially if accompanied by fatigue or infections.
2. Unusual or Rapid Bruising/Bleeding
- Nosebleeds
- Gum bleeding
- Red spots on skin
These often indicate low platelets.
3. Continuous Fatigue, Paleness, or Breathlessness
Common early signs of anemia from bone marrow failure.
4. Severe Bone or Joint Pain
Especially in children (leg pain is common).
5. Swollen Lymph Nodes
Neck, armpits, groin, or abdomen.
6. Abnormal CBC Report
If a routine blood test shows:
- Low hemoglobin
- Low platelets
- High or low WBC
- Blast cells
Evaluation is urgent.
7. Children Becoming Irritable, Tired, or Refusing to Walk
Often an early sign of bone marrow infiltration.
8. Patients Previously Treated With Chemo/Radiation
They need quick evaluation for new symptoms.
How Is B-ALL Diagnosed?
Diagnosis is multi-step and precise.
1. Complete Blood Count (CBC)
Shows:
- Low hemoglobin
- Low platelets
- Abnormal WBC count
- Presence of blasts
2. Peripheral Blood Smear
Examines blood under microscope.
3. Bone Marrow Aspiration & Biopsy
The confirmatory test.
Helps determine:
- Blast percentage
- Immaturity of cells
- Disease subtype
4. Flow Cytometry
Identifies B-cell markers:
- CD19
- CD10
- CD22
- CD34
- TdT
This differentiates B-ALL from T-ALL or other cancers.
5. Cytogenetic & Molecular Testing
Critical for treatment planning.
Tests for:
- BCR-ABL1 (Philadelphia chromosome)
- TEL-AML1 fusion
- Hyperdiploidy
- IKZF1 deletion
- KMT2A rearrangements
These define risk category and predict outcomes.
6. Lumbar Puncture
Checks if leukemia has spread to brain/spinal fluid.
7. Imaging
Ultrasound or CT if organ enlargement suspected.
Types of B-ALL
1. Standard-risk B-ALL
Favorable genetics and good response to treatment.
2. High-risk B-ALL
Genetic mutations, high WBC, or poor early response.
3. Philadelphia Chromosome-Positive (Ph+ B-ALL)
Caused by BCR-ABL1 fusion gene; needs targeted therapy.
4. Philadelphia-Like B-ALL (Ph-Like)
High-risk subtype resembling Ph+ B-ALL but without BCR-ABL1.
5. Early Pre-B / Pre-B / Mature B-ALL
Defined by stage of B-cell development.
Treatment of B-ALL
Treatment is long-term and delivered in phases.
For children: 2–3 years
For adults: Intensive shorter protocols + maintenance
Phase 1: Induction Therapy (First 4 Weeks)
Goal → Achieve complete remission (CR) by eliminating visible leukemia cells.
Includes:
- Vincristine
- Steroids (Prednisone/Dexamethasone)
- Asparaginase
- Anthracyclines (Daunorubicin)
Success rate: 80–95% remission in children, 70–85% in adults.
Phase 2: Consolidation / Intensification Therapy
Goal → Kill residual leukemia cells not visible in tests.
Drugs used:
- High-dose Methotrexate
- Cytarabine
- Cyclophosphamide
- Thioguanine
- Etoposide
Patients are monitored for toxicity, infections, and organ function.
Phase 3: CNS-Directed Therapy
Leukemia can hide in the brain and spine.
So, CNS prophylaxis is mandatory.
Methods:
- Intrathecal chemotherapy (Methotrexate/Cytarabine)
- Occasionally low-dose cranial radiation (rare today)
Phase 4: Maintenance Therapy
Goal → Prevent relapse.
Duration → 18–24 months
Medicines:
- 6-Mercaptopurine
- Methotrexate
- Periodic Vincristine & Steroids
Advanced & Targeted Therapies
a. Tyrosine Kinase Inhibitors (for Ph+ B-ALL)
TKIs target BCR-ABL1.
Examples:
- Imatinib
- Dasatinib
- Ponatinib
These dramatically improve survival.
b. Immunotherapy
1. Blinatumomab (BiTE Therapy)
Redirects T-cells to kill leukemia cells.
Very effective for:
- Minimal residual disease (MRD)
- Relapsed B-ALL
2. Inotuzumab Ozogamicin
An antibody-drug conjugate targeting CD22.
When Is CAR-T Cell Therapy Considered in B-ALL?
CAR-T cell therapy (CD19-directed) is considered when:
1. Disease is Refractory (Not Responding to Chemotherapy)
CAR-T offers high remission rates even when chemo fails.
2. Relapse After Initial Treatment
Especially early relapse within 2 years.
3. Relapse After Stem Cell Transplant
One of the strongest indications for CAR-T.
4. Persistent MRD After Multiple Treatments
Even small traces of leukemia can cause relapse.
5. High-Risk Genetics
Ph-like or KMT2A rearrangements may benefit from early CAR-T.
6. Patient Not Fit for Intensive Chemotherapy
CAR-T is a strong option for medically fragile patients.
Bone Marrow Transplant (BMT)
BMT is recommended for:
- High-risk B-ALL
- Persistent MRD
- Relapsed disease
- Poor induction response
- Ph+ B-ALL with suboptimal TKI response
Transplant types Dr. Bhargava performs:
- Matched sibling
- Matched unrelated donor
- Haploidentical (half-matched)
Prognosis and Survival
Prognosis depends on:
- Age
- Molecular subtype
- Early treatment response
- MRD status
- Overall health
Children:
Very good prognosis → Cure rates 80–90%
Adults:
Moderate prognosis → Cure rates improving due to:
- TKIs
- Blinatumomab
- Inotuzumab
- CAR-T
- Advanced transplant techniques
Frequently Asked Questions
Yes. Children have excellent cure rates (up to 90%) and Adults have significantly improved outcomes with targeted drugs and CAR-T therapy.
Typically 2–3 years. Adults may complete intensive phases faster but still require maintenance.
MRD means a very small number of leukemia cells remain even after treatment.
- MRD is one of the strongest predictors of relapse.
- MRD-negative patients have far better long-term survival.
BMT is recommended when:
-
Leukemia is high-risk
-
There is relapse
-
MRD persists
-
Poor response to initial therapy
-
High-risk genetic mutations are present
Modern advancements include:
-
Blinatumomab: highly effective for MRD+ and relapse
-
Inotuzumab: targets CD22 leukemia cells
-
CAR-T therapy: life-changing option for refractory B-ALL
-
Tyrosine Kinase Inhibitors: for Ph+ B-ALL
These treatments have dramatically improved survival in difficult cases.